Not that long ago, myopia was routinely considered a common, benign refractive error that could be “corrected” with single vision spectacles or contact lenses and remained on the margins of scientific investigation until 2012.1 Thanks to subsequent and significant research into myopia’s prevalence, etiology, risk factors, and more, we now have a greater understanding of myopia, including its potential long-term effects if untreated.1-4
Bullimore et al. illustrated why each diopter matters in myopia control.2 His research and that of others illustrate the risks that high levels of myopia can pose, including the development of myopic maculopathy, glaucoma, retinal tears and detachments, and cataracts.1,5,6
Along with research, industry has kept pace with innovative treatments. CooperVision’s MiSight® 1 day, the first and only soft contact lens designed for myopia control in age-appropriate children, recently marked a milestone: its fifth anniversary of FDA approval.*†7
In a recent white paper, CooperVision’s Priscilla Chang, OD, FAAO, IACMM, details the science behind MiSight® 1 day and key findings behind its 7-year study, which represents the longest, continuous soft contact lens study for myopia control in children.
Here are some key points about ActivControl® Technology and the science behind MiSight® 1 day from Dr. Chang’s paper.
A Closer Look at ActivControl® Technology
ActivControl® Technology was designed to reduce the eye elongation rate during the myopic progression phase in age-appropriate children with myopia.†7 This is important, since slowed progression over multiple years can lead to an accumulated decrease in the magnitude of final myopia, which is expected to reduce the lifetime risk of developing vision-threatening pathological complications as mentioned earlier.2
ActivControl® Technology’s dual-focus optical design consists of alternating concentric zones of different dioptric powers that create two focal planes in the eye — one for clear distance vision, and another that focuses light in front of the retina (myopic defocus). Unlike multifocal optics for presbyopia, dual-focus lenses have discrete power zones with abrupt changes between them. The distance center zone corrects the refractive error, and the surrounding concentric rings of power alternate between treatment zones with approximately +2.00D of myopic defocus, and an additional distance correction zone. The correction zone contains the lens’s labeled power.
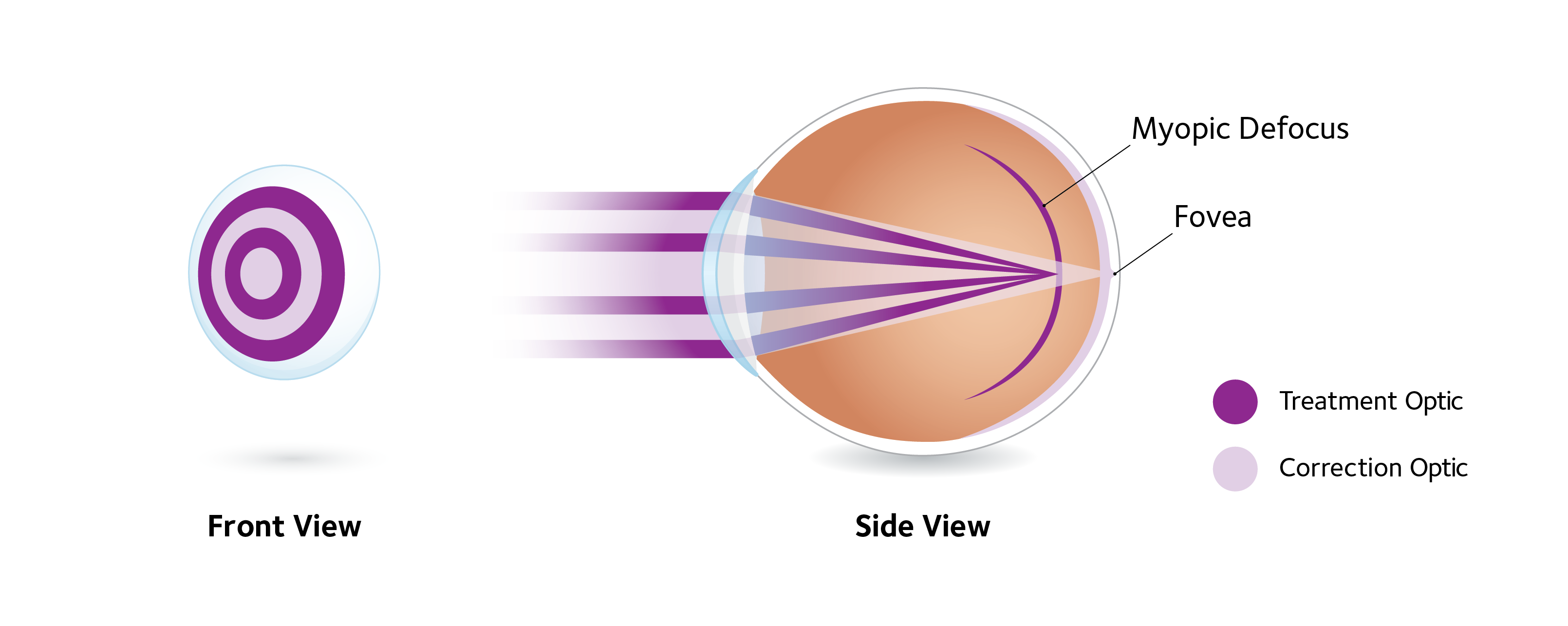
Of note, ActivControl® Technology’s innovative design helps slow eye elongation rates while correcting vision, †7 allowing children to thrive in their daily activities with clear and comfortable vision.7-9
Significant Results from MiSight®’s Clinical Trial
MiSight® 1 day’s 7-year study consisted of three parts:
Part 1 (years 1-3) was a 3-year, multi-center, double-masked, randomized clinical trial that enrolled 144 children aged 8-12. Participants were assigned to wear MiSight® 1 day or a single vision lens (Proclear® 1 day). MiSight® 1 day with ActivControl® Technology slowed the progression of myopia in age-appropriate children by 59% on average, and 41% of the MiSight® 1 day group showed no meaningful progression in refractive error after the first 3 years.†‡§7
Part 2 (years 3-6) included 108 participants now aged 11-15. All participants wore MiSight® 1 day for 3 years. At year six, 23% percent of eyes wearing MiSight® 1 day had no progression.‡§10 On average, children wearing MiSight® 1 day progressed less than -1.00D over the 6-year period‡10
Part 3 (year 7) included 83 participants, now aged 14-18, who wore Proclear® 1 day. The 7-year international MiSight® 1 day clinical study results indicated that there was no clinically significant rebound effect with MiSight® 1 day contact lenses.◊11,12 After treatment cessation, eye growth returned to age-expected levels and the accumulated myopia control treatment gains were retained over 12 months after treatment stopped.◊11,12
Safety, Wearing Success, and Other Key Outcomes
Additional study results illustrated that MiSight® 1 day has a strong safety profile as a daily disposable contact lens,13 and that children are successful in learning how to put in and take out their contact lenses on their own.¶14
Other notable study findings include:
Children as young as 8 years old demonstrated successful handling and confidence soon after initial fitting.**7
At six years, researchers reported no serious adverse events related to contact lens wear,13 suggesting that with proper management and education, pediatric patients can safely wear contact lenses with minimal risk of significant ocular complications.13,15
While the clinical trial protocol instructed participants to wear the lenses for at least 10 hours per day, children consistently exceeded this requirement, wearing their lenses for 13-14 hours daily throughout the study.13
Throughout the clinical trial, over 94% of children reported that they either “don’t notice” or “sometimes notice” the lenses on their eyes,*16 indicating a high level of comfort, and nearly 90% of age-appropriate children preferred MiSight® 1 day contact lenses over their glasses.8
A survey conducted with 8- to 12-year-old children who were new to contact lens wear revealed that 57% found handling the lenses to be “kind of easy” or “really easy” after one week, which improved to 85% by 1 month.8 This figure rose to 97% from six months through the end of the 36-month study.13
Further Reading
You can read or download the complete ActivControl® white paper here. Also, discover more myopia control resources, including MiSight® 1 day’s QuickStart™ program, at CooperVision’s Online Success Center.