You’ve likely heard the exciting news that CooperVision has received U.S. Food and Drug Administration (FDA) approval* of its MiSight® 1 day contact lens, indicated to slow the progression of myopia (nearsightedness or short-sightedness) in children aged 8-12 at the initiation of treatment.
What you may not know is that CooperVision has been involved with myopia research for the past 15 years. One significant area of research has been the multi-site, international, double-masked, randomized study comparing the MiSight® lens to a control lens (the Proclear® 1 day lens). Some of that 5-ear data was presented at the Academy of Optometry Meeting in Orlando earlier this month by Baskar Arumugam, et al in a paper presentation entitled Myopia Progression in Two Matched groups of Children Wearing Dual-Focus Contact Lenses.
For the first three years, this study had a control cohort (which wore the Proclear® 1 day lens) and the test cohort (which wore the MiSight® lens). After the 3 year mark, results indicated that use of MiSight® 1 day was shown to slow myopia progression: 59% as measured by mean cycloplegic spherical equivalent (SE) and 52% as measured by mean axial elongation of the eye.1,2 At that time, the control group was switched into the test lens (the MiSight® 1 day lens) so the entire study cohort was wearing the MiSight® 1 day lens. At this stage of the trial both cohorts of the study are now fitted with the MiSight® lens. The 5 year results of this study were recently presented at the American Academy of Optometry in Orlando, Florida and we’ve summarized the key messages from this poster below.
Both cohorts in years 5 show the same rate of axial length (Figure 1) and myopia progression (Figure 2) and the change rates year upon year are not different between those treated for 2 years (control group) vs. those that have now been treated for 5 years (test group). This suggests that future treatment efficacy does not depend on past treatment history.
Additionally, children can successfully wear MiSight® 1 day contact lenses with minimal impact on ocular physiology ,6 and achieve excellent visual acuity , . The study will continue for another 2 years, 1 year with the subjects still wearing the MiSight® lens and 1 year after cessation of MiSight® lens wear to investigate rebound effects in adolescent.
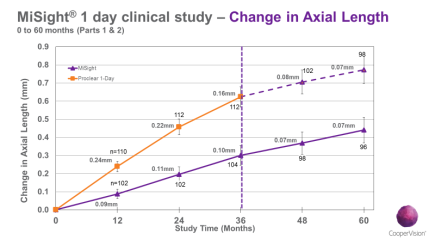
Figure 1. Axial Length Change over 5 Years.

Figure 2. Change in Cycloplegic Spherical Equivalent Refractive Error (SERE) over 5 Years.
So, what does all this mean for your practice?
There is now an FDA-approved* contact lens product indicated to slow the progression of myopia (nearsightedness or short-sightedness) in children aged 8-12 at the initiation of treatment: MiSight® 1 day the first and only of its kind, CooperVision’s commitment to optometry and the area of myopia management is not limited to this commercial product so please check out the additional resources below for further information. This is an active area of research for us and we’re committed to support you by providing resources and insights in the field of myopia management.
To learn more about pediatric myopia and to sign up to receive updates about MiSight® 1 day in the U.S., go to https://coopervision.com/practitioner/ecp-viewpoints/myopia-management
To read the peer-reviewed, open-access, publication on the 3 year results of the MiSight® clinical trial, go to: https://journals.lww.com/optvissci/Fulltext/2019/08000/A_3_year_Randomized_Clinical_Trial_of_MiSight.3.aspx








